While most conjugated polymers within the group are designed to be processed from organic solvents and function as redox materials within organic electrolytes, there is an active push towards conjugated polymers with aqueous compatibility, where the materials are either redox-active in aqueous electrolytes or processable from aqueous-based inks. In the Reynolds group, we have developed aqueous-compatible redox-active materials through the design and study of oligoether-functionalized dioxythiophene-based conjugated polymers. Additionally, we have developed families of polyelectrolyte inks, accessed by side chain defunctionalization, which are suitable for industrially relevant processing.
We reported an oligoether-functionalized conjugated polymer [ProDOT(OE)-DMP] prepared using direct hetero(arylation polymerization).1 This material proves useful in a variety of applications, exhibiting comparable electroactivity in aqueous and organic electrolyte systems, redox stability for thousands of cycles, an ION/OFF current ratio of 105 in accumulation-mode organic electrochemical transistors (OECT) devices, impressive electrochromic contrast (Δ%T > 70%), and capacitance values exceeding 80 F/g.
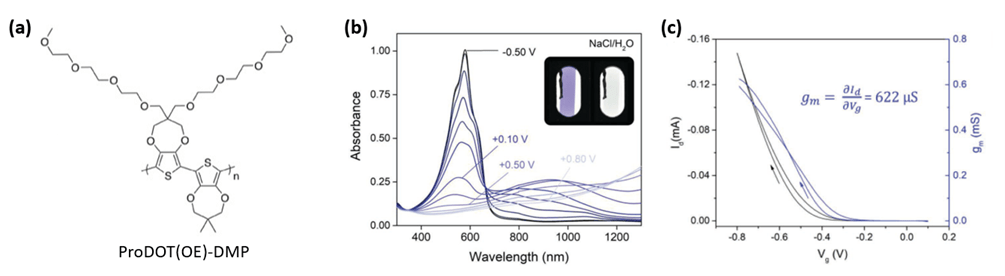
In this work, we address the nature of electrochemically induced charged states in conjugated polymers [ProDOT(OE)-DMP], their evolution as a function of electrochemical potential, and their coupling to their local environment by means of transient absorption and Raman spectroscopies synergistically performed in situ throughout the electrochemical doping process.2
We characterize the coupling of the charge transfer-like state with primary excitons and electrochemically induced charge-separated states, providing insight into the energetic landscape of a heterogeneous polymer-electrolyte system and demonstrating how such coupling depends on environmental parameters, such as polymer structure, electrolyte composition, and environmental polarity.
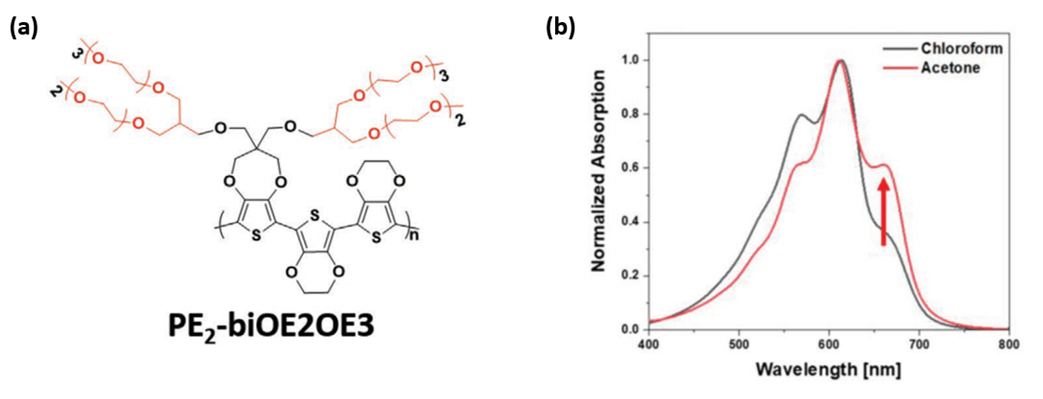
A new dioxythiophenes copolymer comprised of 2,2’-bis-(3,4-ethylenedioxy)thiophene (biEDOT) and 3,4-propylenedioxythiophene (ProDOT) substituted with branched oligo(ether) side chains (PE2biOE2OE3) is synthesized using two direct hetero(arylation) polymerization techniques.3 The typical DHAP results in a lower molecular weight polymer which is soluble in acetone. Alternatively, a unique temperature ramp DHAP methodology results in a higher molecular weight polymer with an especially high solid-state conductivity. Notably, the first OECT fabricated from an acetone-processed polymer is reported which is stable up to 500 cycles and can provide a pathway for future material design aimed at eliminating the use of toxic chlorinated solvents in OECT active layer processing.
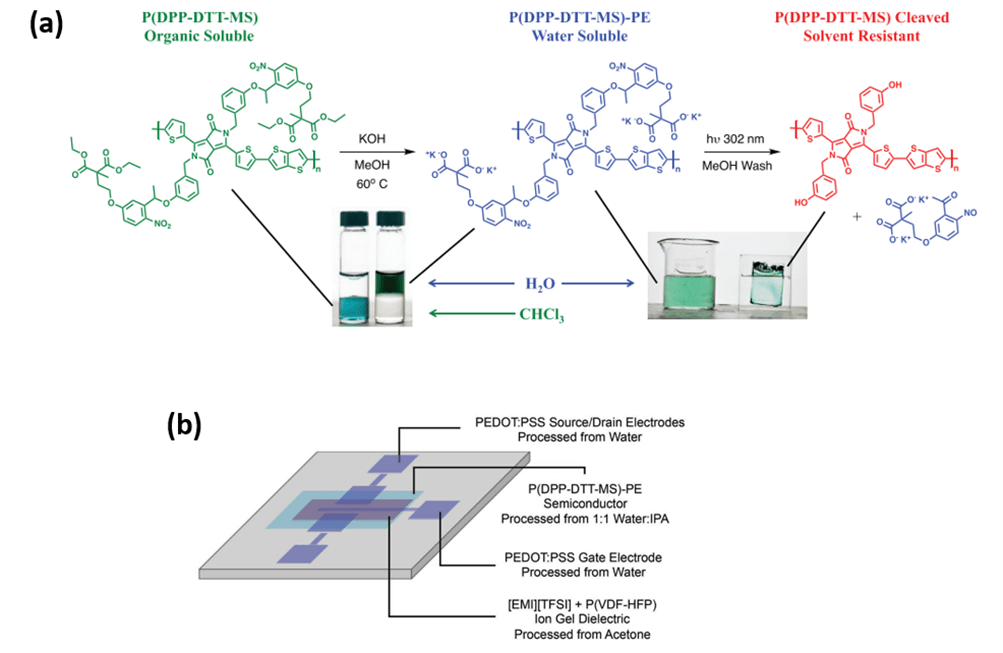
The Reynolds group has additionally utilized a side chain defunctionalization approach to achieve aqueous compatible materials.4,5 A side chain defunctionalization approach within the group uses a combination of ester functionality and o-nitrobenzyl functionality in what is referred to as a multistage side chain approach.4 These polymers are synthesized in organic solvents and can be hydrolyzed to aqueous-processable polyelectrolytes. After casting films from aqueous solutions, the polymer films are irradiated with UV light to remove the side chain mass and render the film insoluble and electroactive. The resulting films can be used in both redox and solid-state applications such as electrochromics and field-effect transistors.
1. Savagian, L. R.; Österholm, A. M.; Ponder, J. F.; Barth, K. J.; Rivnay, J.; Reynolds, J. R. Balancing Charge Storage and Mobility in an Oligo(Ether) Functionalized Dioxythiophene Copolymer for Organic- and Aqueous- Based Electrochemical Devices and Transistors. Advanced Materials 2018, 30, 1804647.
2. Bargigia, I.; Savagian, L. R.; Österholm, A. M.; Reynolds, J. R.; Silva, C. Charge-Transfer Intermediates in the Electrochemical Doping Mechanism of Conjugated Polymers. Journal of the American Chemical Society, 2020.
3. Jones, A. L.; de Keersmaecker, M.; Savagian, L. R.; DiTullio, B. T.; Pelse, I.; Reynolds, J. R. Branched Oligo(Ether) Side Chains: A Path to Enhanced Processability and Elevated Conductivity for Polymeric Semiconductors. Advanced Functional Materials, 2021, 31, 2102688.
4. Schmatz, B.; Lang, A. W.; Reynolds, J. R. Fully Printed Organic Electrochemical Transistors from Green Solvents. Advanced Functional Materials, 2019, 29 (44), 1–7.
5. Ponder, J. F.; Österholm, A. M.; Reynolds, J. R. Conjugated Polyelectrolytes as Water Processable Precursors to Aqueous Compatible Redox Active Polymers for Diverse Applications: Electrochromism, Charge Storage, and Biocompatible Organic Electronics. Chemistry of Materials, 2017, 29 (10), 4385–4392.
