Design and synthesis of new molecules and polymers with unique optoelectronic properties is an ongoing effort in the Reynolds group. Materials are designed and tuned by controlling the chemical structure to access a range of optoelectronic properties.
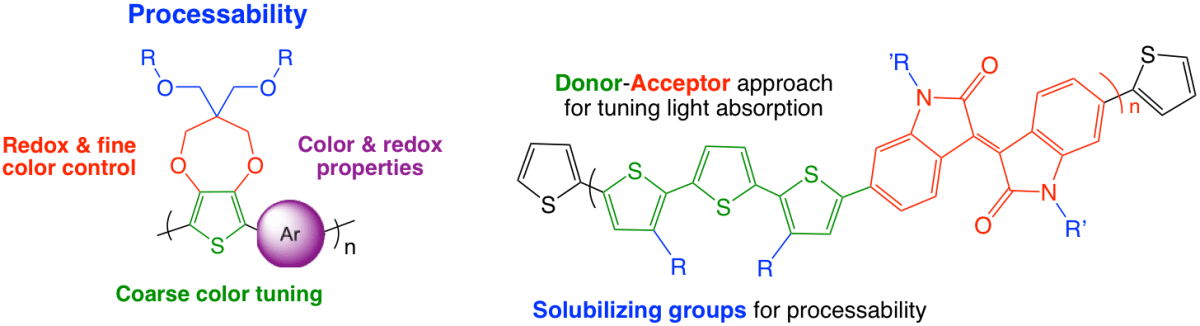
The group has historically relied on Suzuki, Stille, and Grignard metathesis type polymerizations to synthesize new materials, and they are still actively used today. Direct heteroarylation polymerization (DHAP), which offers a fast, clean, and economical way to create many of the same materials, has become our route of choice especially in dioxythiophene and donor-acceptor chemistry for the development of new redox materials for both solid-state and redox applications.1-4
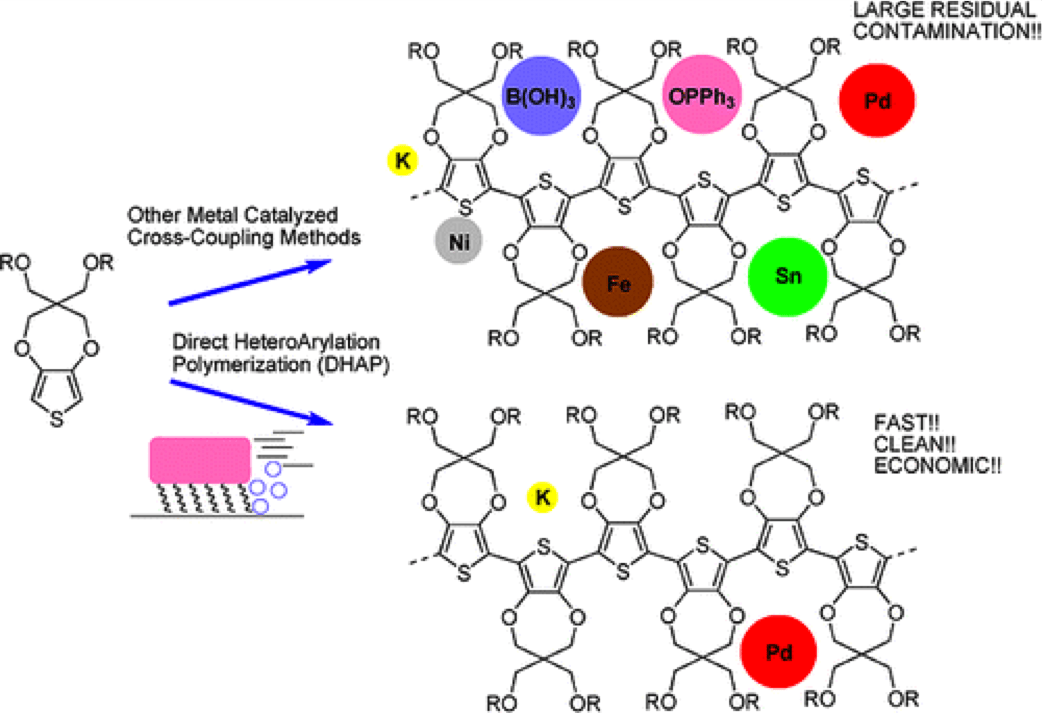
Schematic showing advantages of DHAP over more classical techniques in conjugated polymer synthesis.
Asymmetric DPP synthesis for Langmuir-Blodgett Films
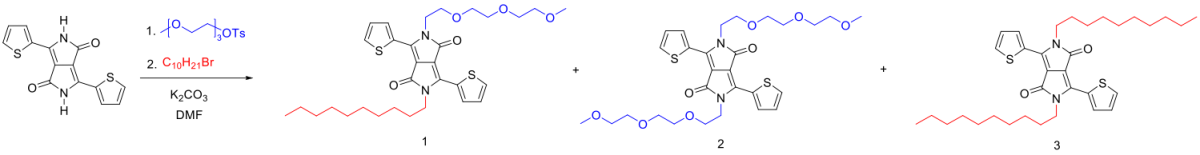
Using a “one-pot synthesis”, we have prepared both asymmetrically and symmetrically substituted diketopyrrolopyrrole (DPP) cores. The reactivity difference between the triglymetosylate and 1-bromodecane in this substitution reaction can be used to strategically control the yield of the three desirable products.5
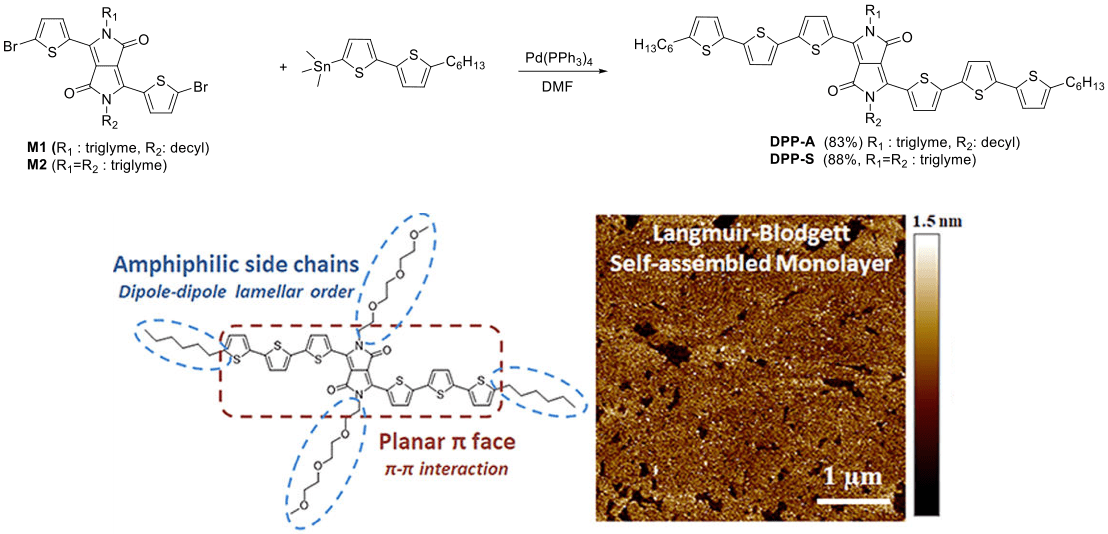
After bromination of the DPP cores and using Pd(PPh3)4 as the catalyst, we synthesize DPP-A and DPP-S via Stillecoupling, both of which are easily purified by recycling column chromatography.
Expanded Side-Chain Selection in Thienopyrrolediones
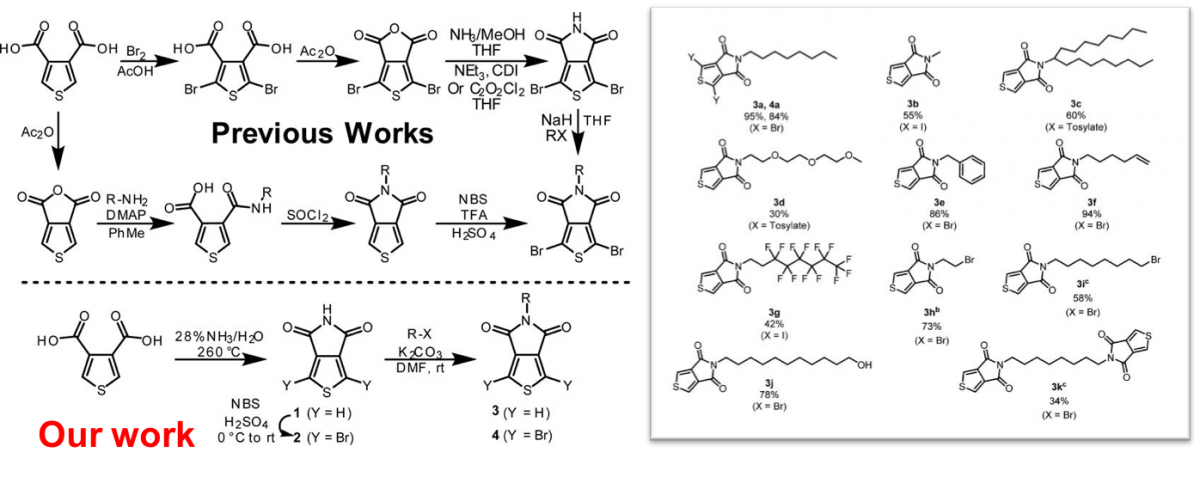
We have redeveloped the synthesis of thienopyrroledione(TPD) to reduce the number of synthetic steps, the amount of byproduct waste, the use of unnecessary solvents, and removed many hazardous and toxic reagents. Diverse functionalization is possible, introducing 16 examples in yields as high as 95%. This reaction scheme is generalizable for thiopheneimides and diverse functionalization is possible, as shown by the 16 examples above with yields as high as 95%.
Chemical defunctionalization can provide water soluble & solvent resistant polymers
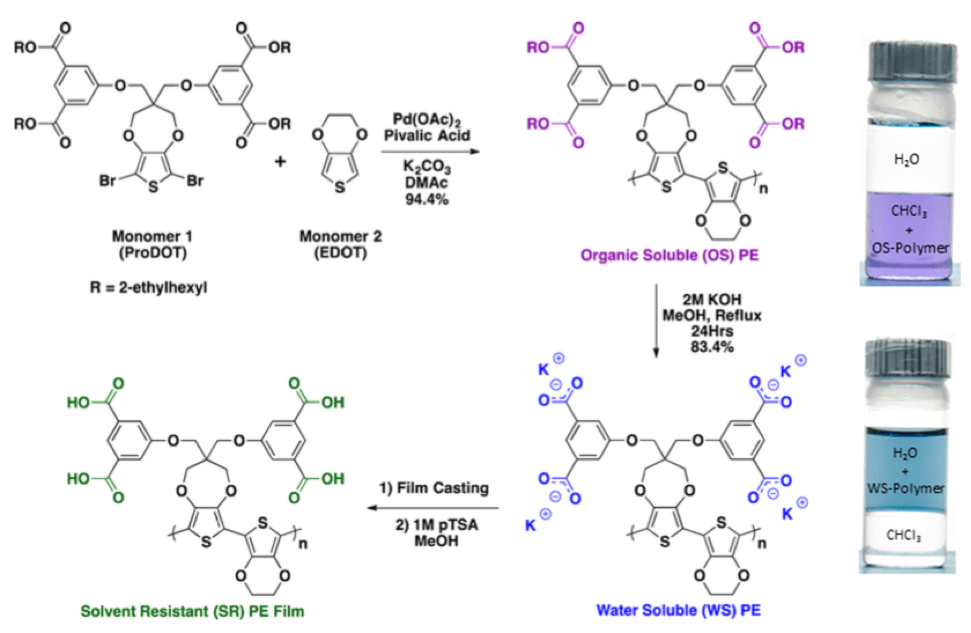
ProDOT can be synthesized with multiple alkyl ester-based side chains. Using this design, upon hydrolysis, the carboxylate that is formed is covalently attached to the polymer backbone. The ester-functionalized monomer is brominated and then polymerized EDOT using DHAP conditions to yield an organic soluble polymer. The ester side chains can be hydrolyzed to form the polymeric carboxylate salt was found to dissolve in water. After deposition, the polymer can be protonated via an acid wash to form a material that is insoluble but highly electroactive.6
1. Kerszulis, J. A., Amb, C. M., Dyer, A. L., Reynolds, J. R. Macromolecules, 2014, 47, 5462.
2. Argun, A. A., Berard, M., Aubert, P.-H., Reynolds, J. R. Adv. Mater. 2005, 17, 422.
3. Amb, C. M., Dyer, A. L., Reynolds, J. R. Chem. Mater. 2011, 23, 397.
4. Estrada, L.A.; Deininger, J.J.; Kamenov, G.D.; Reynolds, J.R. ACS Macro Lett, 2013, 2, 869.
5. Lo, C. K. et. al. ACS Appl. Mater. Interfaces,2018, 10, 11995.
6. Ponder, J. F., Jr.; Österholm, A. M.; Reynolds, J. R. Chem. Mater.2017, 29, 4385.